Abstract
Introduction: The initial response to standard induction chemotherapy for acute myeloid leukemia (AML) is commonly assessed with day 14 bone marrow evaluation; patients with residual disease may receive re-induction chemotherapy. The benefit of re-induction chemotherapy in patients with indeterminate day 14 bone marrow results (defined in our study as ≤ 20% cellularity and 5-20% blasts) remains unclear. We report our experience of AML patients with indeterminate day 14 bone marrow treated with and without re-induction chemotherapy.
Methods: A retrospective chart review was performed to evaluate adult patients with newly diagnosed AML treated with and without re-induction chemotherapy for indeterminate day 14 bone marrow results between January 2010 and April 2018 at our institution. All patients included in the analysis received induction chemotherapy with cytarabine and an anthracycline. Approval for the study was obtained from the Institutional Review Board.
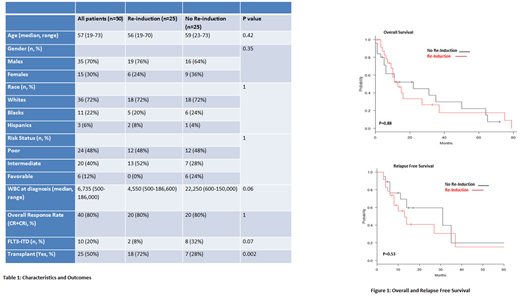
Results: We identified 50 patients with indeterminate day 14 bone marrow results (Table 1). Twenty five patients (50%) received re-induction therapy and 25 (50%) did not. Flow cytometry data on day 14 bone marrow samples were available for 15 patients (60%) who received re-induction and 18 (72%) who did not. All flow cytometry samples, when performed, were reported as persistent/residual disease. The median age at diagnosis was 56 years (range 19-70) in patients who received re-induction and 59 years (range 23-73) in those who did not. There were 12 patients (48%) with poor risk disease, both in the re-induction arm (10 abnormal karyotype, 2 normal karyotype with molecular abnormalities) as well as in the no re-induction arm (6 abnormal karyotype, 6 normal karyotype with molecular abnormalities). FLT3-ITD mutation was positive at diagnosis in 2 cases (8%) in the re-induction arm and in 8 cases (32%) not receiving re-induction. The most commonly used chemotherapy regimen in the re-induction arm was fludarabine, high dose cytarabine and granulocyte-colony stimulating factor (FLAG, 56%). Fifteen patients (60%) in the re-induction arm and 17 (68%) in the no re-induction arm received post-remission consolidation therapy. High-dose cytarabine was the preferred consolidation regimen (96%).
The overall response rate [complete remission (CR) + CR with incomplete count recovery (CRi)] was similar in both arms (80% vs. 80%). There was no statistically significant difference in median overall survival (OS) and relapse free survival (RFS) between the re-induction and no re-induction arms (Figure 1). In univariate analysis, no statistically significant difference in OS was found for age at diagnosis (≥ 60 years vs. < 60 years; p=0.06), white blood cell count at diagnosis (≥ 50,000 vs. < 50,000; p=0.48), disease risk status (poor risk vs. favorable/intermediate risk; p=0.81) and performance of transplant (yes vs. no; p=0.13) in the entire population.
Conclusion: Our study did not find a statistically significant difference in overall response rates, as well as for OS and RFS in AML patients with indeterminate day 14 bone marrow receiving re-induction or not. Our findings question the utility of immediate re-induction chemotherapy and raise the concern for over treatment in this patient population. Larger studies investigating similar outcomes are warranted to validate these clinical findings.
Costa:Celgene: Honoraria, Research Funding; Abbvie: Research Funding; Amgen: Honoraria, Research Funding; Janssen: Research Funding; Karyopharm: Research Funding; Sanofi: Honoraria; BMS: Research Funding. Saad:Actinium: Consultancy. Papadantonakis:Agios pharmaceuticals: Honoraria, Other: advisory board. Erba:Janssen: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; MacroGenics: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Juno: Research Funding; Amgen: Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Daiichi Sankyo: Consultancy, Research Funding; Agios: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding; Astellas: Research Funding; Takeda/Millenium: Research Funding; Janssen: Research Funding; Celgene: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Juno: Research Funding; Amgen: Research Funding; Immunogen: Consultancy, Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Celgene: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Pfizer: Consultancy, Other: grant; Astellas: Research Funding; Agios: Consultancy, Speakers Bureau; MacroGenics: Consultancy; Immunogen: Consultancy, Research Funding; Juno: Research Funding; Celgene: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Takeda/Millenium: Research Funding; Astellas: Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Takeda/Millenium: Research Funding; Amgen: Research Funding; Amgen: Research Funding; Novartis: Consultancy, Speakers Bureau; Janssen: Research Funding; Jazz: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Takeda/Millenium: Research Funding; Janssen: Research Funding; Immunogen: Consultancy, Research Funding; MacroGenics: Consultancy; Pfizer: Consultancy, Other: grant; Agios: Consultancy, Speakers Bureau; Juno: Research Funding; MacroGenics: Consultancy; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Other: grant; Celgene: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Pfizer: Consultancy, Other: grant; Jazz: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Immunogen: Consultancy, Research Funding; Astellas: Research Funding; Incyte: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal